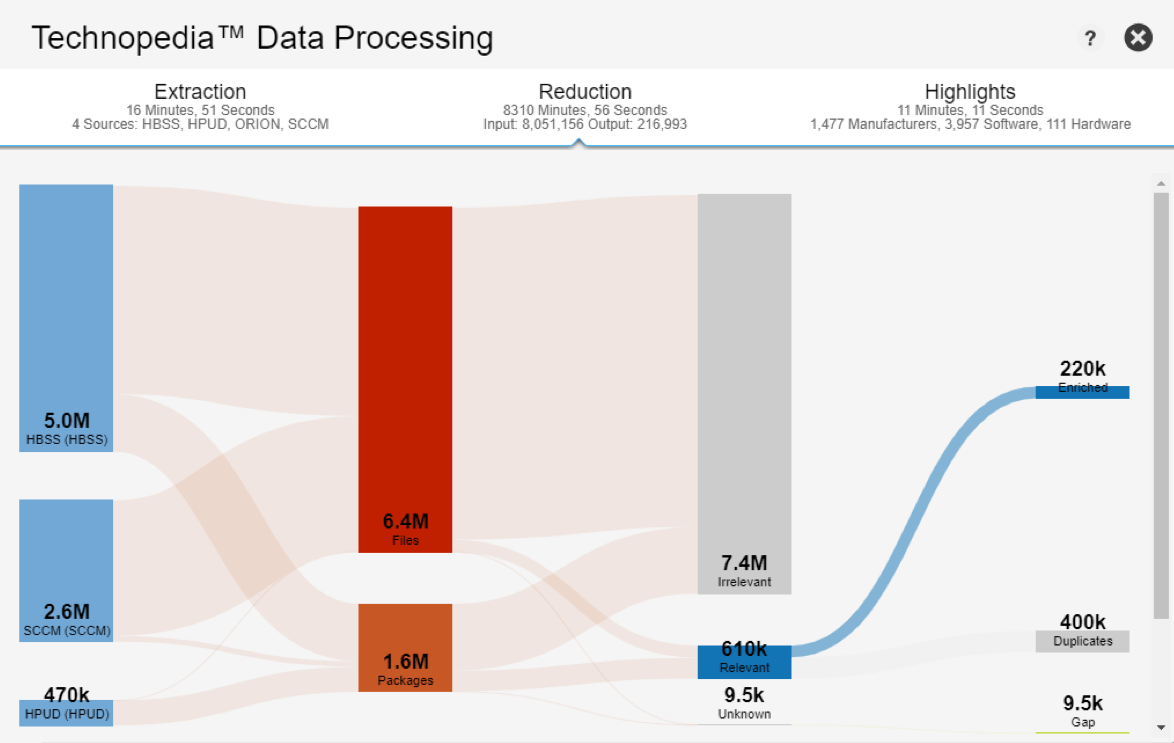
Data Platform
Gain insight into your technology environment
Visualize all your IT asset data for better decision making.
Benefits
Detailed info on 3.9+ million standardized hardware and software models
- Clean data with full transparency on data journey, from source to result
- Complete visibility into your assets allowing actionable analysis to support IT asset management initiatives
- Simplified vendor audit process and reduced cost of true-ups
- Reduced IT costs, increased service levels and customer satisfaction
- Tight control on software and hardware currency to ensure compliance with regulatory requirements
- Detailed info on 3.9+ million standardized hardware and software models
- Comprehensive source of technology catalog, enabling enterprise architecture initiatives to build a perfect asset portfolio
- Content packs extend catalog with up-to-date market data, and daily updates ensure data fidelity
- Awareness of software security landscape with advisories from Secunia Research
Featured Details
Make your data do more
Normalize for a common language and enrich for intelligent action.
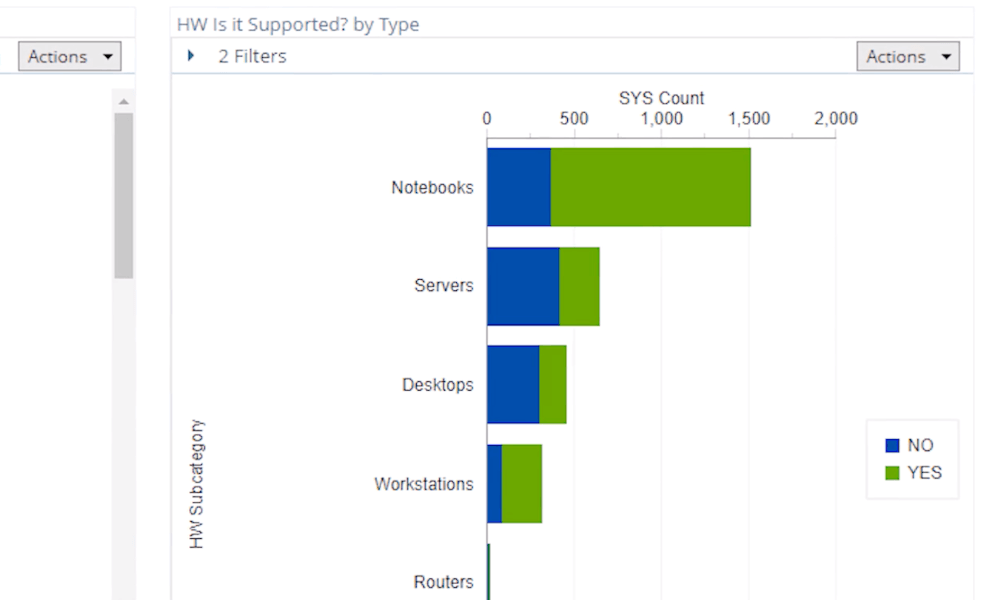

Is your IT asset management at risk?
24%
feel they have complete visibility into IT assets
50%
cite vulnerabilities as their greatest concern
46%
operating systems are vulnerable by EOL/EOS
*Figures have been rounded to the nearest integer.
Informing IT, Transforming IT
Industry insights to help keep you informed
White Papers & Reports
Forrester Research: The Total Economic Impact of the Flexera Data Platform
Learn how you can achieve 302% or more ROI by using Flexera Data Platform. Read this Forrester Consulting study today!
Datasheets
The quality data you need for better IT asset decisions
You need quality data to make good decisions—and Flexera’s Data Platform delivers. See how you can get clean, accurate and complete data for your enterprise architecture and technology initiatives.
Webinars
How to drive business value and establish an award-winning ITAM program
Join former Nike IT executive Jason Patterson to hear critical insights on how to deliver business value and establish an optimized ITAM program.
Case Studies
Financial institution’s enriched SAM data pays strategic business dividends
Northern Trust, a leading financial institution, wanted to design their IT program so that it would work collaboratively with the company’s business decision makers. They chose the Flexera One suite of ITAM and FinOps solutions that delivered enriched SAM data to achieve their strategic alignment goals.
Case Studies
A Textbook Case of Improving Asset Data Management for this Connecticut University
Find out how asset management solutions from Flexera solved both hardware and software management issues.
Blog
The power of normalization: A key to unlocking IT efficiency
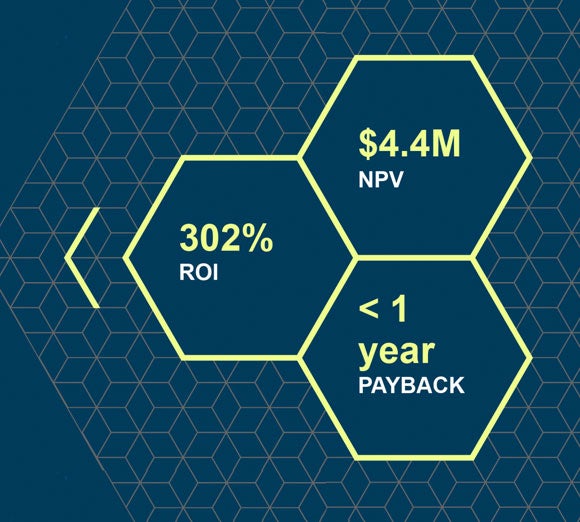
The Total Economic Impact™ of the Flexera Data Platform
See the high-level results of a Forrester® study on the three-year financial impact of using our data platform.

Request Your Data Platform Demo
Schedule a demo to see how Flexera Data Platform can help you gain a complete view of your IT ecosystem.